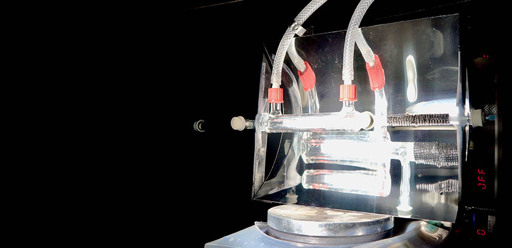
Here is the link to the study.
The researchers, from the University of Cambridge, say their solar-powered reactor could be used to make fuel to power cars and planes, or the many chemicals and pharmaceuticals products we rely on. It could also be used to generate fuel in remote or off-grid locations.
Unlike most carbon capture technologies, the reactor developed by the Cambridge researchers does not require fossil-fuel-based power, or the transport and storage of carbon dioxide, but instead converts atmospheric CO2 into something useful using sunlight. The results are reported in the journal Nature Energy.
Carbon Capture and Storage (CCS) has been touted as a possible solution to the climate crisis, and has recently received £22bn in funding from the UK government. However, CCS is energy-intensive and there are concerns about the long-term safety of storing pressurised CO2 deep underground, although safety studies are currently being carried out.
A tree?
We did it. We made a robo tree 😄
No, tress die and when they rot they release the carbon. You cannot plant enough trees to stop climate change unless you wipe most of humanity off the planet at the same time. We must remove the carbon we introduced into the cycle when we started using fossil fuels.
This is why carbon sequestration is important to pursue.
You might be interested in taking a look at the following articles:
Reality check on technologies to remove carbon dioxide from the air | MIT, Nov 2024
Study finds many climate-stabilization plans are based on questionable assumptions about the future cost and deployment of “direct air capture” and therefore may not bring about promised reductions.
The fossil fuel industry promotes solutions such as carbon capture and storage, liquefied natural gas, hydrogen, and renewable natural gas, which critics argue are far more focused on preserving industry profits than significantly reducing greenhouse gas emissions. The tactics they use include funding university research that skews public discourse and policymaking in the direction of their preferred solutions. They have hired management consultancies to conduct skewed analysis supporting those solutions and funded lobbyists, and advertising and public relations firms to promote them.
im not saying we need to make it into fuel
You charcoal all trees and brush and put it back into the soil. Helps with water retention and promotes healthy soil. You can put it in cattle feed and reduce methane production. Plus, all that carbon is stable for hundreds of years. I have a small cone pit where I do small batches. My tomatoes and flowers seem to like it.
Well, you could always:
- Chop down the tree
- Turn it into charcoal
- Bury the charcoal deep under ground
That way, the carbon in the tree is removed from the cycle and no longer re-emitted.
It’s been done before. And it’s not like trees quickly die and they don’t rot quickly either.
(For those not in the know, Kahn killed so many people, allowing farmlands to revert to forest, that the carbon sequestration lowered temperatures. https://news.mongabay.com/2011/01/how-genghis-khan-cooled-the-planet/)
pre-industrial examples aren’t going to be relevant just due to the concentration of carbon consumed continuously over that time.
Ya lost me. ?
Just killing a lot of people and letting the trees naturally return womt reverse it because we have put so much carbon into the cycle. Everyone burning fires is nothing compared to an industrialized nation
It’s making syngas (mix of hydrogen and CO).
So it begs for a next step. Syngas on its own is not a practical fuel. Unlike methane, it can’t be liquefied (hydrogen does not liqefy under economically feasible conditions). Due to free hydrogen, it makes metals brittle. Due to CO, it’s poisonous. And like most fuels, it’s flammable - I think we can’t blame a fuel for that. :)
The next step is hydrogenation of carbon monoxide. I’ve browsed literature and read my fair share via Sci-Hub, and this step tends to have various issues: reactivity, selectivity, catalyst cost and catalyst lifetime.
Reactivity: often, you have to raise either the pressure or the temperature to levels which complicate industrial production. Directly reacting CO2 with H2 faces those issues, but the catalyst (Cu + ZnO) is cheap.
Selectivity: suppose you want to get methanol, the simplest alcohol. Unfortunately your catalyst gives you a mix of methane, methanol, ethane, ethanol and buthanol. To build an industrial process, you need an extra step to separate them. If you get too much byproducts, your fuel production plant could become considerably bigger and more costly. So you definitely want good selectivity.
Catalyst lifetime: suppose that 1 kg of catalyst manages to produce 100 kg of fuel. That’s nice in a lab, but clearly unaccepable in industry.
Catalyst cost: for example, you better not need appreciable quantities of rare metals (e.g. rhenium, nice catalyst, but 2500 euros per gram).
Recently, much has been written about hydrogenation of CO in its liquid phase (at high pressure, not low temprature). For example here. The catalyst is manganese (price OK) and the “total turnover number” (representing catalyst lifetime) is 12 000, which I’d describe as “good enough to go out of the lab, if cheap enough”. In the summary, I can’t find their batch time. In another study about CO + H2 via manganese, people used a batch time of 8-12 hours. So there is a reactivity issue present, but maybe it can be overcome.
They’re going to feel pretty silly when they realise people are already powering cars from solar by just charging the car.
Alternative solutions like fossil-free fuels are worth looking into because batteries are heavy.
Not better than BEVs, public transport or better batteries until they prove it’s practicable, just worth at least looking at it.
But but no middleman to profit! Won’t you think of the middleman!
Lobbyists will create a middleman, always.
I think they’re aiming for storing and transporting a large amount of energy (e.g. enough energy for the whole winter). Which you conventionally do as fuel.
Cyberpunk is becoming real